 Israeli startups unveil nontoxic technologies to vanquish head lice. Photo illustration by Lightspring/Shutterstock.com
Israeli startups unveil nontoxic technologies to vanquish head lice. Photo illustration by Lightspring/Shutterstock.com
Israeli startups have invented poison-free solutions for an itchy problem, one using vaporized vinegar and the other ultrasound waves.
The global head-lice epidemic is a real head-scratcher.
In the United States alone, up to 12 million children get infested with head lice each year. Across the world, the head-lice treatment market has reached $1.8 billion worth of special shampoos, conditioners and combs promising to vanquish the little wingless parasites.
Combing is tedious and may leave some lice or eggs behind, while hair products aren’t always effective against resistant “super lice” and can contain strong chemicals.
Two Israeli companies – TechCare and ParaSonic – have innovative nontoxic approaches to tackling this itchy problem.
TechCare
TechCare of Rosh HaAyin is soon launching Novokid in Israel. The kit contains disposable capsules of a natural vinegar-based solution, which are connected to a vaporizing device that blows the dry particles into a hair cap, suffocating the lice within 10 minutes. No shampooing is necessary.
The patented system is based on research by graduates of Israel’s Weizmann Institute of Science and is expected to receive CE approval for Europe shortly and FDA approval for the United States in 2018.
“We have a robotic manufacturing line at our production site so we can ramp up to mass production quickly,” says TechCare CEO and Chairman Zvi Yemini, a serial entrepreneur and chairman of Israel’s Shenkar College of Engineering and Design.
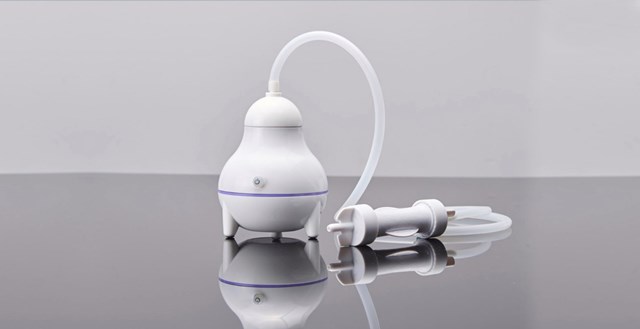
The Novokid vaporizer blows a dry vinegar solution into a hair cap. Photo courtesy of TechCare
About $4 million was invested into the development, testing and commercialization of Novokid. “We’ve achieved high effectiveness rates and extremely positive reviews,” says Yemini.

A repeat treatment or weekly treatment is recommended to catch any remaining or newly hatched lice and to prevent reinfestation. The kit, containing the cap, machine and four capsules, will sell for about $49, while a pack of four capsules will be $12-$15.
A second product, Shine, a hair conditioning device utilizing the same technology, is set for global release following regulatory approval later this year. The company is also developing additional applications of its technology platform.
TechCare plans to open an office in New Jersey. The headquarters and manufacturing will remain in Israel. “All the companies I invest in, even when sold, stay in Israel,” says Yemini, whose previous ventures include ZAG Industries (bought by Stanley Black & Decker), Hydro Industries (bought by Suncast) and Polymer Logistics. He’s also on the board of hot startups Aquarius Engines and Nano Dimension.
ParaSonic
ParaSonic has developed a patented comb-like device featuring rows of teeth made of electrical creosote ceramic material that produces ultrasonic waves meant to destroy head lice and their eggs in a single combing. The mechanism does not allow for the lice to develop resistance as they do to chemicals.

ExLicer. Photo: courtesy
The company, based on a patented IP by entrepreneur Mor Cohen, was incubated in 2014 at NGT3 in Nazareth and has raised $1.6 million from a private American investor on top of ₪3 million from NGT3 and the Israeli Innovation Authority.
“When Mor approached us with the idea, we did due diligence and learned the market is very large and there is a great need for better alternatives,” says Zohar Gendler, CEO of NGT3. “There are many techniques for destroying lice, but destroying eggs is complicated and destroying both is even more complicated.”
ParaSonic is managed by former fighter pilot David Tavor, former CEO of companies including CURE Medical, Hadas Natural Products, and Fluorinex, which was acquired last year by Colgate.
Tavor expects a “reasonable” regulatory pathway for ParaSonic’s device. “We are in the process of completing clinical trials at Herzliya Medical Center. In parallel to the trial, we redesigned the device and we estimate that at the end of June we’ll be able to proceed toward production of prototypes and be in the market by the first quarter of 2018,” he tells ISRAEL21c. “We’ll focus on Israel and the US initially and have started the process of getting FDA and CE approvals. Interest is enormous.
