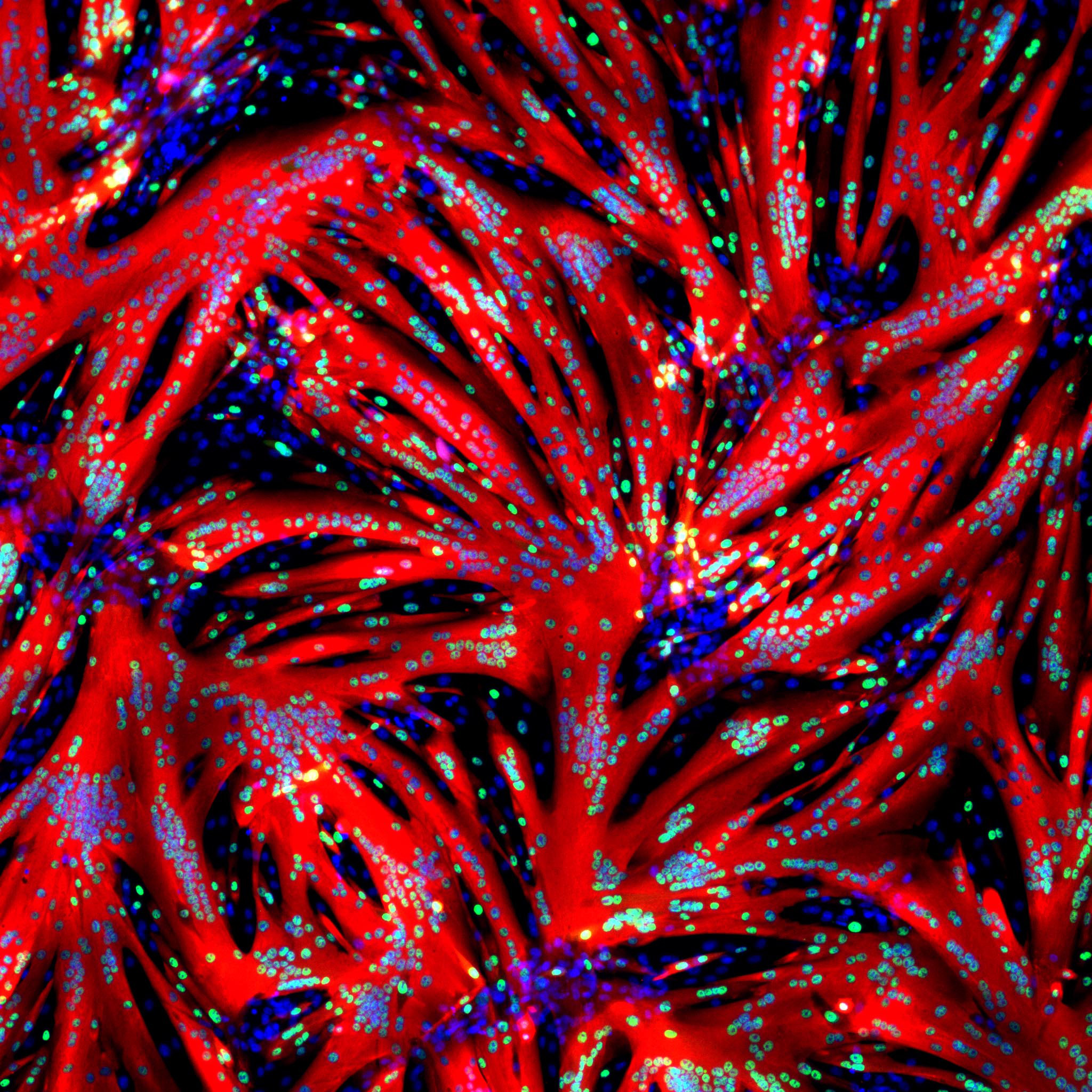
REHOVOT, ISRAEL—December 20, 2021—Prof. Eldad Tzahor peered into his microscope one day and saw steak. As part of Tzahor’s research into repairing muscle tissue, Dr. Tamar Eigler, a postdoctoral fellow in his lab at the Weizmann Institute of Science, had been experimenting with cultured muscle stem cells. One of these experiments had produced the surprising sight that appeared before Tzahor’s eyes: The cells had started fusing into tiny fibers that thickened rapidly, within hours creating large muscle fibers resembling those in whole cut meat.
To unravel the chain of molecular events behind this rapid transformation, Tzahor and Eigler, both of the Molecular Cell Biology Department, teamed up with Dr. Ori Avinoam of the Biomolecular Sciences Department, who studies cell-to-cell fusion. That chain begins with the exposure of muscle stem cells, called myoblasts, to a small molecule that blocks an enzyme termed ERK. The blockage causes these cells to start differentiating and fusing into tiny fibers, and it leads to the activation of another enzyme, CaMKII, which ends up triggering a massive myoblast fusion and expansion. These findings are being published today in Developmental Cell.
“Since all muscles in our bodies and those of other animals, including cattle, are produced by the same biological processes, our findings may be applicable both to the study of muscle regeneration and to the production of cultivated meat,” Tzahor says.
Myoblasts are formed in the embryo, but a tiny fraction of these cells stays on top of muscle fibers throughout our lives, even though their number declines with age. When a muscle is injured, these stem cells are the ones responsible for its repair and regeneration. To initiate the repair process, these cells must stop dividing so they can mature and start fusing with one another and with the injured muscle tissue.
“Figuring out what regulates the fusion of myoblasts is crucial for understanding muscle repair,” Eigler says. “Without fusion there is no regeneration.”
Experiments led by Eigler indeed suggest that the newly discovered pathway leading to myoblast fusion might be one involved in muscle regeneration. When Eigler created genetically engineered mice that lacked the end enzyme of this pathway, CaMKII, these mice were slower to repair a muscle injury than those whose bodies produced the enzyme.
The new approach has enabled the scientists to watch how fusion progresses over time. Their observations support the notion that fusion occurs in distinct primary and secondary stages—something that was previously shown in fruit flies but not in vertebrates.

“In the primary stage, single-nucleus myoblasts fuse together to form myotubes, which have two or three nuclei,” Avinoam explains. “In the secondary stage, fusion goes into high gear, as the rest of the myoblasts are drawn to fuse with the already formed myotubes, creating muscle fibers that contain dozens or even hundreds of nuclei. Most of the fusion takes place during this secondary stage.”
Time-lapse videos show that the fusion process enters the secondary stage twelve to sixteen hours after the cells are exposed to the ERK-blocking molecule. That’s when the process suddenly takes off, speeding up enormously. Within the next twelve hours or so, the fibers furiously fuse into meaty muscle.
The fact that all the myoblasts in a laboratory dish start fusing in a synchronized manner suggests that they may be following a built-in program for making muscle. And this, in turn, suggests that the processes observed in the lab closely mimic the way muscle fibers fuse in the body.
In follow-up experiments, the researchers showed that the ERK-to-CaMKII pathway drives muscle differentiation and fusion in cultured myoblasts taken from several species of farm animals, including chickens, cows and sheep. The study’s findings may, therefore, help speed up the production of cultivated meat, thereby reducing its cost.
A new startup company, ProFuse Technology, was launched recently to develop the findings for use in the food tech industry. Yeda Research and Development Company, the Weizmann Institute’s technology transfer arm, has granted the company exclusive rights to this technology and to the patent covering this research.
There’s a historic Weizmann Institute angle to this research: One of the founding fathers of the study of muscle growth, the late Prof. David Yaffe, had been a Weizmann scientist. The myoblast cultures that he developed in the 1960s for exploring the differentiation and fusion of these cells have for decades been used by scientists all over the world. Now, some sixty years later, the new, efficient way of inducing such differentiation and fusion discovered by Weizmann scientists may greatly advance future studies in the field.
Study participants included Giulia Zarfati, Dr. Sansrity Sinha and Dr. Nadav Segev from Avinoam’s lab; Emmanuel Amzallag, Avraham Shakked and Dr. Kfir-Baruch Umansky from Tzahor’s lab; Yishaia Zabary and Dr. Assaf Zaritsky of Ben-Gurion University of the Negev; Dr. Eyal D. Schejter of Weizmann’s Molecular Genetics Department; and Dr. Douglas P. Millay of the University of Cincinnati College of Medicine.
Prof. Eldad Tzahor is Head of the Yad Abraham Research Center for Cancer Diagnostics and Therapy; his research is also supported by Mr. and Mrs. Israel Englander.
Dr. Ori Avinoam is the incumbent of the Miriam Berman Presidential Development Chair; his research is also supported by the Yeda-Sela Center for Basic Research; the Henry Chanoch Krenter Institute for Biomedical Imaging and Genomics; and the Schwartz/Reisman Collaborative Science Program.
